Sold out
Formic acid, glass ROTIPURAN® ≥98 %, p.a., ACS
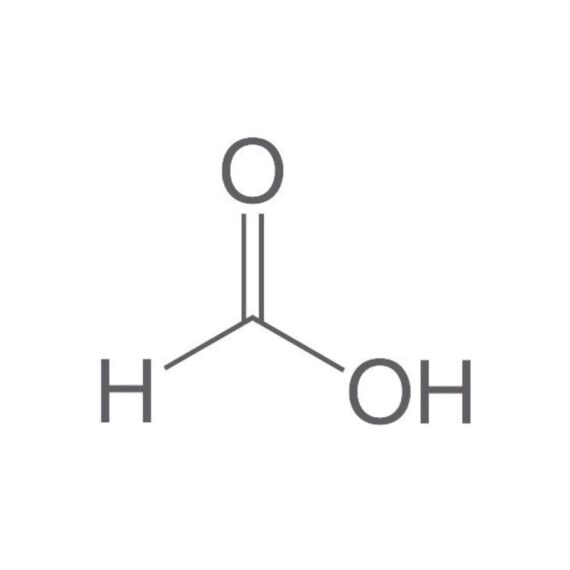
Formic acid
Formylic acid, Methanoic acid, Hydrogen carboxylic acid
Empirical formula CH2O2
Molar mass (M) 46,02 g/mol
Density (D) 1,22 g/cm³
Boiling point (bp) 101 °C
Flash point (flp) 49 °C
Melting point (mp) 4 °C
ADR 8 II
WGK 1
CAS No. 64-18-6
EG-Nr. 200-579-1
UN-Nr. 1779
Molar mass (M) 46,02 g/mol
Density (D) 1,22 g/cm³
Boiling point (bp) 101 °C
Flash point (flp) 49 °C
Melting point (mp) 4 °C
ADR 8 II
WGK 1
CAS No. 64-18-6
EG-Nr. 200-579-1
UN-Nr. 1779
 |
Examples of effect: Flammable; liquids form compounds with the air which can become explosive; produce flammable gases with water or can self-combust. Safety: Keep away from open flames and sources of heat; close containers tightly; store in a fire-safe manner. |
 |
Examples of effect: Damage metals and burn body tissues; may cause serious eye damage. Safety: Avoid contact; wear safety spectacles and gloves. In the event of contact with eyes and skin, rinse with water. |
 |
Examples of effect: In smaller quantities, lead to immediate serious damage to health or death. Safety: Do not inhale, touch or swallow. Wear work safety equipment. Call a poison control centre or doctor immediately. Recovery position. |
| H226-H290-H302-H314-H331-EUH071 | flammable liquid and vapour, may be corrosive to metals, harmful if swallowed, causes severe skin burns and eye damage, toxic if inhaled |
| P210 P280 P303+P361+P353 P304+P340 P305+P351+P338 P310 | keep away from heat, sparks, open flames, hot surfaces. No smoking, wear protective gloves/eye protection/face protection, IF ON SKIN (or hair): Take off immediately all contaminated clothing. Rinse skin with water [or shower], IF INHALED: Remove person to fresh air and keep comfortable for breathing, IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continue rinsing, immediately call a POISON CENTER/doctor. |
Description
Formic acid
Further attractive products to complete your chromatography laboratory can be found on our Chromatography page!
Determination of Viscosity
Viscosity is the measure of a fluid’s resistance to flow. The lower a liquid’s viscosity, the thinner it is and the more easy it is to pour; the higher a liquid’s viscosity, the thicker it is and the harder it is to pour. The particles in viscous liquids are less mobile as they have to overcome the van der Waals forces and the bonds between the particles in them are stronger.
Acids and Bases for Analysis (p.a.)
Carl ROTH provides suitable acids and bases for different requirements!
- High purity
- High batch consistency
- Detailed specifications
- Good price-performance ratio