Sodium hydroxide solution 0,33 mol/l – 0,33 N, volumetric standard solution
Bs. 0.00Bs. 0.00
Sodium hydroxide solution, 0,33 mol/l – 0,33 N, volumetric standard solution
Empirical formula NaOH
Molar mass (M) 40,01 g/mol
Density (D) 1,015 g/cm³
Boiling point (bp) 100 °C
ADR 8 III
CAS No. 1310-73-2
EG-Nr. 215-185-5
UN-Nr. 1824
| Amount-of-substance conc. (20 °C) | 0,33 mol/l ± 0,2 % |
| Titer | 0,998-1,002 |
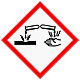 |
Examples of effect: Damage metals and burn body tissues; may cause serious eye damage. Safety: Avoid contact; wear safety spectacles and gloves. In the event of contact with eyes and skin, rinse with water. |
| H290-H315-H319 | may be corrosive to metals, causes skin irritation, causes serious eye irritation |
| P280 P302+P352 P305+P351+P338 |
wear protective gloves/eye protection, IF ON SKIN: Wash with plenty of water, IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continue rinsing |
Description
Sodium hydroxide solution, 0,33 mol/l – 0,33 N, volumetric standard solution
Volumetric Standard Solutions
A volumetric standard solution is a solution containing a precisely known concentration of a substance. The concentration of a volumetric standard solution is determined using a primary standard, which is accurately weighed and dissolved to make a specific volume. Volumetric standard solutions are made using reagent-grade (p.a.) substances as source materials.
Volumetric Standard Solutions, ready-to-use
The advantages of this over making them yourself are:
- Manufactured and tested using modern manufacturing and analytical methods
- Ready-to-use solutions
- High accuracy for precise analyses
- Application of NIST-certified reference substances for checking
- Connection to standard titrators is possible